PCB design is a crucial element in the development of medical devices, serving as the foundation for their functionality and performance. As medical technology advances, ensuring that PCBs meet stringent regulatory standards is essential for patient safety and device reliability. Compliance with standards such as the FDA and IEC 60601 guarantees that medical devices are safe, effective, and meet the necessary performance requirements.
Medical devices must meet high standards for safety and performance. To achieve this, designers must ensure that their PCB designs comply with strict regulations set by the Food and Drug Administration (FDA) and IEC 60601 standards.
Table of Contents
- The Role of PCBs in Medical Devices
- Compliance with Regulatory Standards
- FDA Compliance for Medical Device PCBs
- IEC 60601 Compliance
- Key Factors in Medical Device PCB Design
- Testing and Validation for Compliance
- IoT in Medical Device PCB Design
- Future Trends in Medical Device PCB Design
- How HashStudioz Can Help with Your PCB Design
- Conclusion
The Role of PCBs in Medical Devices
PCBs are essential in nearly every medical device, from diagnostic tools to life-saving equipment. These devices depend on accurate, reliable, and high-performance PCBs to function. A malfunctioning PCB can cause a device to fail, which may endanger patient health. For example, in life-support devices, a PCB failure could lead to catastrophic outcomes. Therefore, it is critical to design medical device PCBs with the utmost precision and reliability.
Compliance with Regulatory Standards
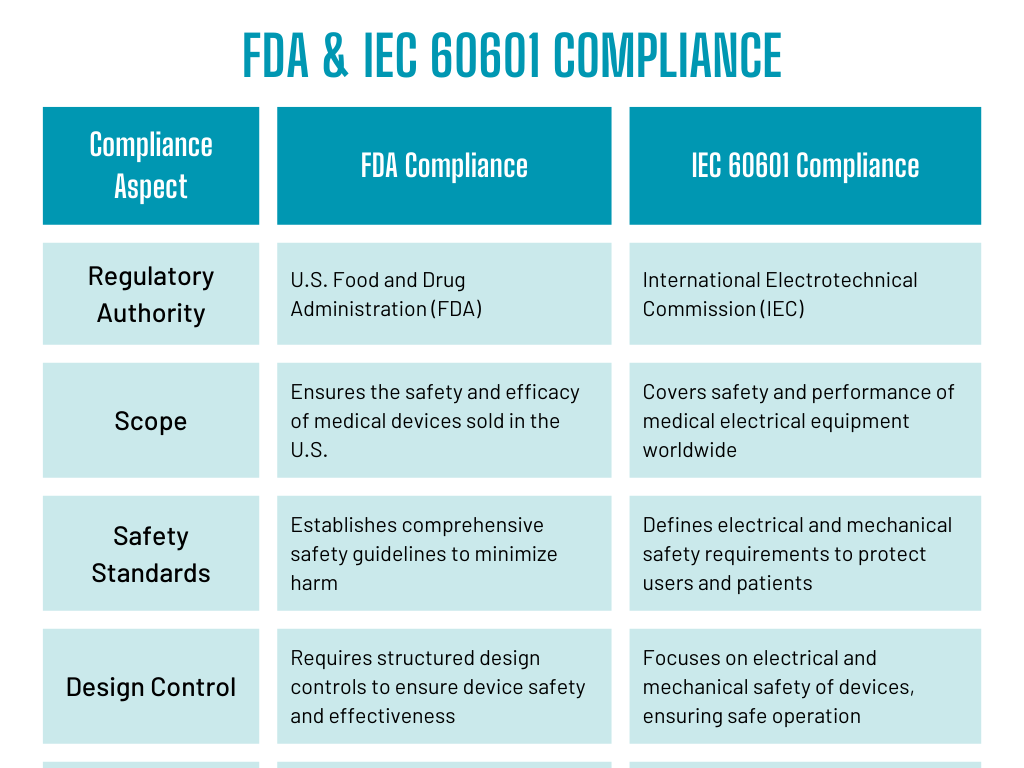
Designing PCBs for medical devices requires compliance with two key sets of regulations: the FDA guidelines and IEC 60601 standards. These regulations ensure the safety, performance, and quality of medical devices.
FDA Compliance for Medical Device PCBs
The FDA oversees the safety and efficacy of medical devices in the United States. Medical device manufacturers must comply with the FDA’s 21 CFR Part 820 regulations. These regulations mandate a comprehensive quality management system (QMS) throughout the device’s life cycle, including PCB design.
Key FDA compliance aspects
- Design Control: This ensures that devices meet their intended purposes and are safe for use.
- Risk Management: Designers must identify potential risks and reduce them through design improvements.
- Performance Testing: Testing is required to ensure that the PCB and device function as intended.
Failure to meet FDA standards can lead to delays, fines, or product recalls. Therefore, FDA compliance should be a priority from the very beginning of the design process.
IEC 60601 Compliance
IEC 60601 is an international standard for the safety and performance of medical electrical equipment. It outlines requirements to prevent electrical hazards, electromagnetic interference (EMI), and other potential risks. Compliance with IEC 60601 is necessary for devices to be sold globally.
Key IEC 60601 requirements
- Electrical Safety: Ensures that the device does not pose any electrical hazards to patients or healthcare providers.
- Electromagnetic Compatibility (EMC): Prevents interference between the medical device and other equipment.
- Environmental Considerations: Devices must function safely under various environmental conditions, such as temperature and humidity fluctuations.
Manufacturers must adhere to IEC 60601 to ensure that their devices meet global safety standards. Non-compliance can prevent entry into international markets.
Key Factors in Medical Device PCB Design
Designing a PCB for a medical device involves several considerations. These factors ensure that the device operates reliably and complies with regulatory standards.
1. Component Selection
Choosing the right components is vital for medical device PCBs. The components must meet reliability, durability, and safety standards. For instance, components should be resistant to environmental factors like moisture and temperature changes. Additionally, they must comply with FDA and IEC 60601 standards to ensure safety and performance.
2. Redundancy and Fail-Safe Mechanisms
Redundancy is essential in life-critical devices. It ensures that if one component fails, the device can continue operating. For example, a redundant power supply circuit or a backup communication channel may be necessary to maintain functionality.
3. Signal Integrity and Power Distribution
Signal integrity is crucial in medical devices. Accurate readings and precise device performance depend on clear signal transmission through the PCB. Designers must also ensure stable power distribution to avoid fluctuations that could impair device performance.
4. Thermal Management
Medical devices can generate heat during operation. Effective thermal management in PCB design is necessary to prevent overheating, which can damage components and affect device performance. Thermal management techniques include using heat sinks, thermal vias, and proper component placement.
5. Miniaturization and Space Constraints
In medical devices, size is often a key consideration. Designers must create compact PCBs that fit within tight spaces while maintaining functionality. Advanced PCB manufacturing techniques enable the miniaturization of components, ensuring that devices remain small, portable, and lightweight.
Testing and Validation for Compliance
After designing the PCB, it is critical to conduct extensive testing. Testing ensures that the device meets regulatory and performance standards.
1. Electrical Testing
Electrical testing verifies that the PCB works as intended. This includes checking for proper voltage levels, signal transmission, and power distribution. Medical devices must also undergo electromagnetic compatibility (EMC) testing to ensure that they do not cause or are affected by electromagnetic interference.
2. Environmental Testing
Environmental testing ensures that the device performs well in different conditions, such as varying temperatures and humidity levels. Testing also simulates potential exposure to dust, moisture, or liquids.
3. Compliance Testing
To meet FDA and IEC 60601 requirements, medical device PCBs undergo compliance testing. This includes electrical safety tests, EMC tests, and performance tests. The device must pass these tests to be deemed compliant and safe for use.
IoT in Medical Device PCB Design
The rise of IoT technology has revolutionized the medical device industry. IoT-enabled devices offer features like real-time monitoring, remote diagnostics, and automated data collection. As a result, the PCB design for these devices must account for additional challenges.
Key considerations for IoT-enabled medical device PCBs include:
- Wireless Communication: PCBs must support wireless technologies like Bluetooth, Wi-Fi, or Zigbee.
- Power Efficiency: Many IoT devices operate on battery power. PCB designs must minimize energy consumption to prolong battery life.
- Data Security: IoT medical devices must protect patient data from cyber threats. This requires secure communication protocols and encryption.
By incorporating IoT capabilities, medical devices can offer enhanced functionality and improve patient outcomes. HashStudioz offers advanced IoT development services to help you design and integrate IoT features into your medical devices while ensuring regulatory compliance.
Future Trends in Medical Device PCB Design
The medical device industry is evolving rapidly, and PCB design is at the forefront of these changes. Several key trends are shaping the future of PCB design for medical devices. These advancements aim to improve device performance, reliability, and integration with emerging technologies, all while maintaining strict regulatory compliance.
1. Miniaturization and Flexible PCBs
Miniaturization remains a central trend in medical device design. As devices become smaller and more portable, PCB designs must follow suit. Flexible PCBs are gaining popularity, especially in wearable medical devices. These PCBs can bend and conform to the shape of the body, allowing for more compact, comfortable, and functional devices.
Flexible designs help maintain performance while reducing device size. Flexible PCBs are critical in devices such as pacemakers, hearing aids, and medical sensors.
2. Integration of IoT and Wearable Devices
IoT technology is revolutionizing the medical device industry. IoT-enabled devices allow real-time data sharing with healthcare providers. This improves patient monitoring and care management. Medical device PCBs will need to support advanced wireless communication protocols like 5G. These technologies will enable faster data transfer, enhancing device performance.
Additionally, wearable medical devices, such as heart rate monitors and glucose trackers, require efficient, compact, and low-power PCBs. IoT integration allows for continuous monitoring, providing more proactive healthcare.
3. Advanced Materials for Enhanced Performance
New PCB materials will improve performance and durability. Advanced substrates such as polyimide and ceramics enhance thermal management and signal integrity. These materials are especially important in devices that operate in harsh environments. Biocompatible materials are also on the rise, particularly for implantable devices. These materials ensure safe interaction with the human body. As medical devices evolve, these advanced materials will be essential in achieving high-performance, long-lasting, and safe products.
4. Artificial Intelligence in PCB Design
Artificial Intelligence (AI) is becoming a valuable tool in PCB design. AI-driven tools help optimize the design process, ensuring faster and more efficient workflows. These tools detect design flaws early, reducing human errors. AI also has the potential to improve predictive maintenance in medical devices. Embedded AI can monitor device health and alert users to necessary maintenance or calibration. This proactive approach can prevent device failure and improve patient safety.
5. 3D Printing and Additive Manufacturing
3D printing and additive manufacturing are transforming PCB production. These technologies allow for the creation of highly customized PCBs. 3D printing can produce PCBs with intricate, multi-layered designs that traditional methods can’t achieve. This is especially valuable in producing patient-specific medical devices. For example, custom implants can be designed to fit a patient’s unique anatomy. These innovations open the door to highly specialized, personalized medical devices.
6. Cybersecurity Integration
As medical devices become more connected, cybersecurity is more critical than ever. IoT-enabled devices must be protected from unauthorized access. Future PCBs will incorporate security features such as encryption and secure boot processes. These security measures will protect sensitive patient data. Ensuring that medical devices comply with data protection regulations, such as HIPAA, is essential. Secure PCBs will help prevent cyber threats and maintain trust in medical technology.
How HashStudioz Can Help with Your PCB Design
At HashStudioz, we specialize in providing high-quality PCB design services for medical devices. Our team of experts understands the complex requirements of the medical industry and is committed to delivering PCB designs that comply with FDA and IEC 60601 standards. We offer end-to-end solutions, from design and prototyping to testing and compliance certification.
Our services include:
- FDA-compliant PCB design: Ensuring all designs meet FDA’s stringent regulations.
- IEC 60601 compliance: Creating medical device PCBs that meet global safety standards.
- IoT-enabled design: Incorporating IoT features for enhanced functionality and remote monitoring.
- Prototyping and Testing: We offer comprehensive prototyping services and thorough testing to ensure reliability and safety.
With HashStudioz, you can trust that your medical device PCBs will meet the highest standards of quality and compliance. Contact us today to learn more about our PCB design services and IoT development services.
Conclusion
Designing PCBs for medical devices is a challenging but crucial task. Compliance with FDA and IEC 60601 standards is essential for ensuring the safety, performance, and reliability of medical devices. By following best design practices, conducting thorough testing, and leveraging the expertise of companies like HashStudioz, manufacturers can create devices that meet regulatory requirements and improve patient care.
If you are looking to develop medical devices with high-quality, compliant PCBs, HashStudioz is here to help. Our PCB design services and IoT development services will ensure that your devices meet all safety and performance standards, enabling you to deliver cutting-edge medical solutions to the market. patient care.
For manufacturers looking to develop high-quality, compliant medical devices, partnering with experienced providers like HashStudioz ensures that your PCB designs meet all the necessary regulatory requirements and leverage the latest technologies. Whether you need PCB design services or IoT development services, our team is here to support your journey toward delivering safe, efficient, and reliable medical devices to the market.

